Characterization of electrokinetic and membrane properties of nanoporous polyimide films via measurements of time-resolved pressure-induced potential
Abstract
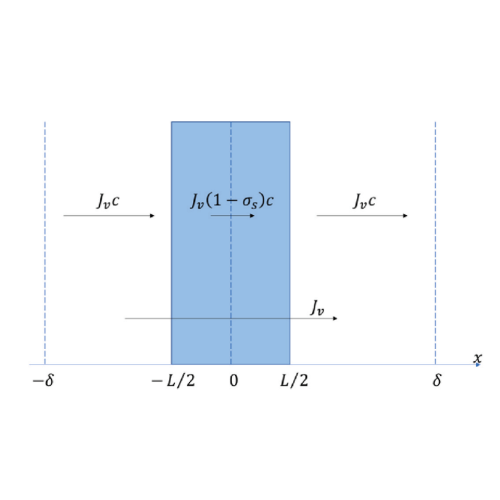
Nanoporous films with charged surfaces exhibit unique ion-separation properties, largely influenced by the surface charge density. Traditionally, this is estimated using the streaming potential. However, in dilute electrolyte solutions, the pressure-induced electrical response is not solely due to streaming potential, but also to concentration gradients from partial salt rejection by charged pores.
We observe that in dilute solutions (<2 mM KCl), the electrical response is primarily driven by the concentration potential rather than the streaming potential, challenging common assumptions. These concentration gradients develop over time, allowing separate measurement of instantaneous (streaming) and time-delayed (concentration) components. The study introduces an improved interpretation method that accounts for non-linear and osmotic effects, providing more reliable results and supporting the development of new applications for nanoporous films.
Explore Further
Full Article: